how many electrons will the lithium atom give up to become stable?
Electron Shells
Electrons are in constant motion outside of an atom's nucleus. The electron shell is the region that the electrons travel in (see Fig. 2.21). Electron shells are labeled with numbers 1 through 7. Each shell holds an increasing number of electrons, beginning with electron shell 1, which holds a maximum of two electrons (see Table 2.6).
| Electron Shell | Number of electrons per shell |
|---|---|
| 1 | 2 |
| 2 | 8 |
| 3 | 18 |
| 4 | 32 |
| 5 | 50 |
| 6 | 50 |
| 7 | 50 |
In theory, electron shells 6 and 7 can hold more electrons, but in the known elements, 50 is the maximum number of electrons in these shells.
When one shell fills to its limit, electrons are added to the next shell. In a neutral atom, the number of negatively charged electrons is equal to the number of positively charged protons.
- Hydrogen (H), with atomic number one, has one electron in shell 1 (Fig. 2.21 A).
- Oxygen (O), with atomic number eight, has a total of eight electrons, two in shell 1 and six in shell 2 (Fig. 2.21 B).
- Sodium (Na), with atomic number eleven, has two electrons in shell 1, eight electrons in shell 2, and one electron in shell 3. (Fig. 2.21 C).
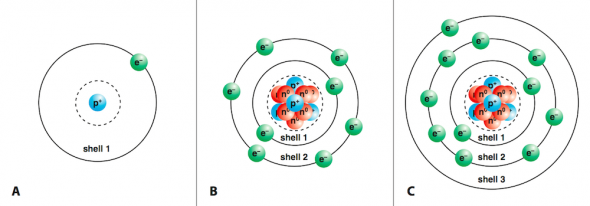
As the electron shells go from 1 to 7, they increase in size and average energy. In other words, the farther the shell is from the nucleus, the larger it is, and the higher its average energy. The valence shell is the outermost electron shell of an atom. In general, the electrons in valence shells determine how the atom behaves in chemical reactions. For example, atoms with complete valence shells, the noble gases, are the least chemically reactive. On the other hand, electrons that have only one electron in their valence shells (Group 1 elements) or elements that are just one electron short of having a complete shell (Group 17) are the most reactive.
Ions
While the atomic number, the number of protons in the nucleus, never changes, some electrons are easily lost or gained by an atom. When an atom gains or loses an electron, the atom no longer has a balanced charge. Therefore, the atom is no longer neutral. An ion is a charged atom. An atom that has gained negatively charged electrons becomes negative. A negative ion or anion is an atom that has gained electrons. An atom that has lost negatively charged electrons becomes positive. A positive ion or cation is an atom that has lost electrons.
Nonmetals tend to gain electrons and become anions. For example, in Fig. 2.22 A, a neutral oxygen atom (O), with eight protons and eight electrons, gains two electrons. This gives it two more negative charges than positive charges and an overall charge of 2–. Metal elements tend to lose electrons and become cations. For example, in Fig. 2.22 B, a neutral sodium atom (Na), with 11 protons and 11 electrons, loses one electron. This gives it one less negative charge than positive charges and an overall charge of 1+.
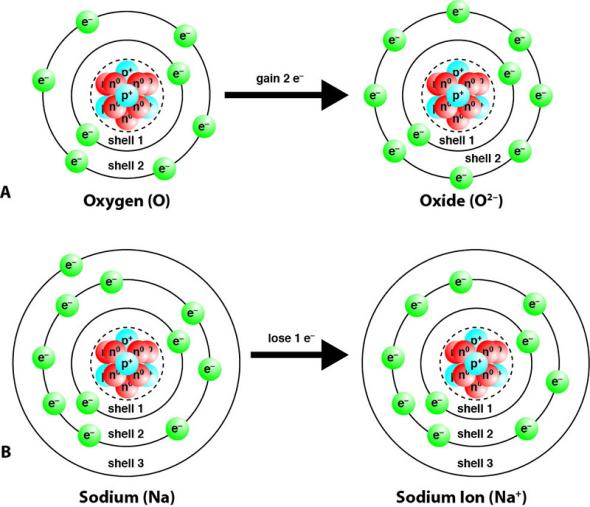
Group 18 elements, the noble gases, are very stable (non-reactive). This is because they have completely full valence electron shells. The octet rule states that regardless of how many electrons a shell can potentially hold, the valence shell can only hold eight electrons. The noble gases have eight electrons in their valence shells. Other elements will gain or lose electrons to achieve completely full valence shells, eight electrons in the valence shell, so that they are also stable. In Fig. 2.22 A, the oxygen atom gained two electrons so that it had eight electrons in shell 2, its valence shell. In Fig. 2.22 B, sodium lost one electron so that it had eight electrons in shell 2, which becomes its valence shell.
The periodic table can help in predicting the type of ion that an element will form based on how many electrons need to be gained or lost for it to become stable. Elements will gain or lose electrons to have the same configuration as a noble gas, in other words, to have a full octet. Atoms tend to gain or lose the least number of electrons to achieve a full octet. In other words, if an atom could lose one electron or gain seven to have a full octet, it will lose one.
Ion Formation Patterns
In general, atoms form ions according to the following patterns:
- Metals in Group 1 have only one electron in their valence shell. They can give up this one electron and become 1+. Group 2 elements give up two electrons to become 2+, and Group 3 give up three electrons to become 3+.
- Nonmetals in Group 17 need just one electron to complete their valence shell. They can gain one electron and become 1–. Group 16 elements gain two electrons to become 2–, and Group 15 elements gain three electrons to become 3–.
- All the remaining metal elements produce at least one ion with a charge of 2+.
Some elements can form ions with two or more different charges. Iron (Fe), for example, can form both an iron ion with 2+ (Fe2+) and an iron ion with 3+ (Fe3+).
Naming Ions
A neutral sodium (Na) atom loses one electron to form a sodium ion (Na+) with a charge of 1+ (see Table 2.8). A positive ion, or cation, has the same name as the element. Thus there are sodium (Na+) ions as well as potassium (K+) ions, calcium (Ca2+) ions, and aluminum (Al3+) ions. Notice that if the charge of an ion is 1+, the symbol is a superscript plus (+), without the number 1 (e.g., Na+).
| Element | Element Name | Protons in neutral atom | Electrons in neutral atom | Electrons gained or lost to achieve octet | Ion formed | Ion Name |
|---|---|---|---|---|---|---|
| Na | Sodium | 11 | 11 | 1 lost | Na+ (cation) | Sodium ion |
| Cl | Chlorine | 17 | 17 | 1 gained | Cl- (anion) | Chloride ion |
A neutral chlorine (Cl) atom that gains one electron changes into a chloride ion (Cl-) with a charge of 1– (see Table 2.8). To name a negative ion, or anion, the last part of the name of the atom is dropped and replaced with -ide. Thus there are chloride ions (Cl-), fluoride (F-) ions, sulfide (S2-) ions, and nitride (N3-) ions. Notice that if the charge of an ion is 1–, the symbol is a superscript minus (-), without the number 1 (e.g., Cl-).
Ionic Compounds
An ionic compound is a compound that is formed by ionic bonding. Ionic bonding occurs through a process called electron transfer, where one atom gives electrons to another. Imagine two puppies. One of the puppies has a bone (Fig. 2.23 A). The puppies represent atoms. The bone represents an electron. The puppies are both neutral. One puppy is a thief and steals the bone from the other puppy. The puppy thief now has the bone (Fig. 2.23 B). The puppy that does not have a bone, that lost its electron and is now positively charged, will follow the thief puppy, which has a negative charge, around to form a puppy pair. The puppy pair is the ionic compound (Fig. 2.23 C).



In electron transfer, an atom of one element loses one or more electrons, and an atom of another element gains those electrons. Both of the atoms involved in electron transfer become ions. The atom that gains the electrons becomes a negatively changed anion, the atom that loses the electrons becomes a positively charged cation. The opposite charges on the ions cause the ions to bond, or be held together, by electrostatic forces. An ionic bond is a bond between ions where oppositely charged atoms attract each other and cancel their charges to produce neutral compounds.
Information about electron shells and ion formation can be used to predict how elements will interact to form ionic compounds. For example, each element in Group 1 gives up one electron to become a 1+ cation. Each element in Group 17 can gain one electron to become a 1– anion. Elements from Groups 1 and 17 can combine to form ionic compounds in a one-to-one ratio. Therefore, one lithium (Li) cation bonds with one fluorine (F) anion as lithium flouride (LiF). Other examples of ionic compounds that combine in a ratio of one cation to one anion are sodium chloride (NaCl) and potassium iodide (KI). In comparison, Group 1 cations (1+) combine with Group 16 anions (2–) in a two-to-one ratio. So, there are two lithium cations for every oxygen anion when they bond to form lithium oxide (Li2O). Bonds between other elements in Groups 1 and 16 also form two-to-one ratios. Examples of these include potassium oxide (K2O), lithium sulfide (Li2S), and sodium sulfide (Na2S).
An example of a one-to-one ratio ionic bond is shown in Fig. 2.24. A sodium atom transfers an electron to a chlorine atom (Fig. 2.24 A). During this process, the sodium has lost an electron to become a positive Na+ cation and chlorine has gained an electron to become a Cl– anion (Fig. 2.24 B). The Na+ ion then bonds to the Cl– ion by electrostatic forces. The 1+ charge of the sodium is balanced by the 1– charge of the chlorine. The resulting sodium chloride (NaCl) compound is neutrally charged (Fig. 2.24 C).



An example of a two-to-one ratio ionic bond is shown in Fig. 2.25 for magnesium and chlorine. Because each magnesium atom can lose two electrons, and each chlorine atom can only gain one electron, magnesium must transfer its two electrons to two chlorine atoms. The magnesium cation (Mg2+) then bonds with two chloride anions (Cl-).



Salts are Ionic Compounds
When most people use the word salt, they mean a specific kind of salt, sodium chloride (NaCl). Sodium chloride is the common table salt that we put on food. However, the term salt has a more general meaning in chemistry; salts are ionic compounds formed of cations and anions held together by ionic bonding.
In a crystal of table salt, sodium and chloride ions are arranged very closely together. A single, tiny crystal of table salt can be composed of a billion trillion ions. The sodium and chloride ions in table salt are arranged very closely together, their arrangement forms a crystal in the shape of a cube. In other salts, the ions may be arranged differently to form crystals of different shapes (Fig. 2.26).


how many electrons will the lithium atom give up to become stable?
Source: https://manoa.hawaii.edu/exploringourfluidearth/chemical/chemistry-and-seawater/ionic-compounds
Posted by: gomezsonsen.blogspot.com

0 Response to "how many electrons will the lithium atom give up to become stable?"
Post a Comment